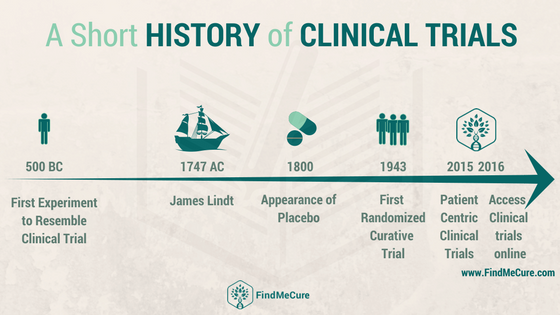
The History of Clinical Trials

When talking about the history of clinical trials one name always pops up – that of James Lind who conducted the famous scurvy trial in 1747. When we were talking about how it all started with the FindMeCure team, we all knew about James Lind but were curious to find out more – to take a look beyond the mythologized story and share our findings with you. Like everything in history, there are many approaches and ways to interpret facts and occurrences, so let’s start our journey to through the centuries of medical research – and since that’s in my line of study, I hope I make a decent tour guide.
Not a clinical trial per se but more of an accidental experiment are the events described in the Biblical “Book of Daniel”, which follows the Babylonian King Nebuchadnezzar in his efforts to keep his subjects healthy by ordering they live on a strict diet of meat and wine. Of course, some of his subjects – the sworn vegetarians – were not too happy with the king’s orders so he gave them permission to keep their diet of vegetables and water for the duration of ten days. At the end of this “test period” the king noticed that the vegetarians were healthier looking than the ones only living off of meat and wine, so he changed his – as we would call it today – public health policy.
Another story, though much better recorded, is that of the surgeon Ambroise Pare who in 1537, having run out of oil – then used for the treatment of wounds, was forced to think on his feet and find a different way to tend to the soldiers he was responsible for. Pare applied on the wounds instead of a mixture of “yolks of eggs, oil of roses and turpentine”. He writes he couldn’t sleep that night, overcame with worrisome thoughts, but to his surprise in the morning he found the soldiers he treated with his experimental mixture feeling much better than the ones who received the last doses of oil.
James Lind And The Scurvy-Healing Citrus

The 18th century blessed Europe with what we now refer to as “The Age of Reason” or Enlightenment. It brought into the spotlight values like intellectualism, logic, and precision. And while it won’t be for another century that the first laboratory and thus, experiments conducted in a controlled environment were born, empirical knowledge had already become a thing.
This brings us to James Lind and his scurvy “clinical trial”. The year is 1747 and Lind, a surgeon on a ship decides to test out the most promising cures for scurvy, describing a process closely resembling our modern day controlled clinical trials. He chose a group of similar cases on one and the same diet and gave different medicine in pairs – cider, vitriol, vinegar, sea water, oranges, and lemons. Of course, as you know, the oranges and lemons “won” with the cider following closely but here’s what happened next: oranges were far too expensive to be recommended as treatment despite the high levels of mortality. It was another 50 years before the British Navy supplied every overseas journey with lemon juice and even then the lemons were eventually replaced by the much cheaper at that time limes.
The Modern Day Situation
There is this trend in social sciences to date phenomena based on their linguistic genesis, or in other words – a thing becomes a thing when there is a word for it. So, while James Lind probably didn’t conceive of his experiment as a “clinical trial” and we are definitely being anachronistic by calling King Nebuchadnezzar’s orders “health policy”, the 19th and 20th centuries came with a significant growth in specialized jargon. And the reason for that is simple – there were more advancements in science than ever before and they required their own “language”. The placebo – a key aspect of the development of clinical trials – was introduced in 1863 but we’re going to skip the 19th century here because the real ethical concerns in medicine came with World War II.
The first double blind controlled trial took place in 1943 testing patulin for the treatment of common cold, which after all though the procedure itself was carried out with precision (a great challenge at wartime) was a failure as patulin turned out ineffective. The trial, however, made history with its execution being the first of its kind.
The years after the War was even more eventful. Though the Hippocratic oath precedes any modern day medical advancement, the horrors of human experimentation under the Nazi regime shook the Western world and called for new laws guiding medical research. The Nuremberg Code of 1947 emphasized voluntarily given consent and this concern about informed consent was further regulated in the US with the 1962 Kefauver-Harris amendments. The Helsinki Declaration of 1964 added specific recommendations for the use of human subjects in clinical trials, thus starting a tradition of updating existing requirements as the practices themselves change every few years. The FDA, founded in 1862, also changed its status gaining more power over drug-related legislature after the 1906 Food and Drugs Act.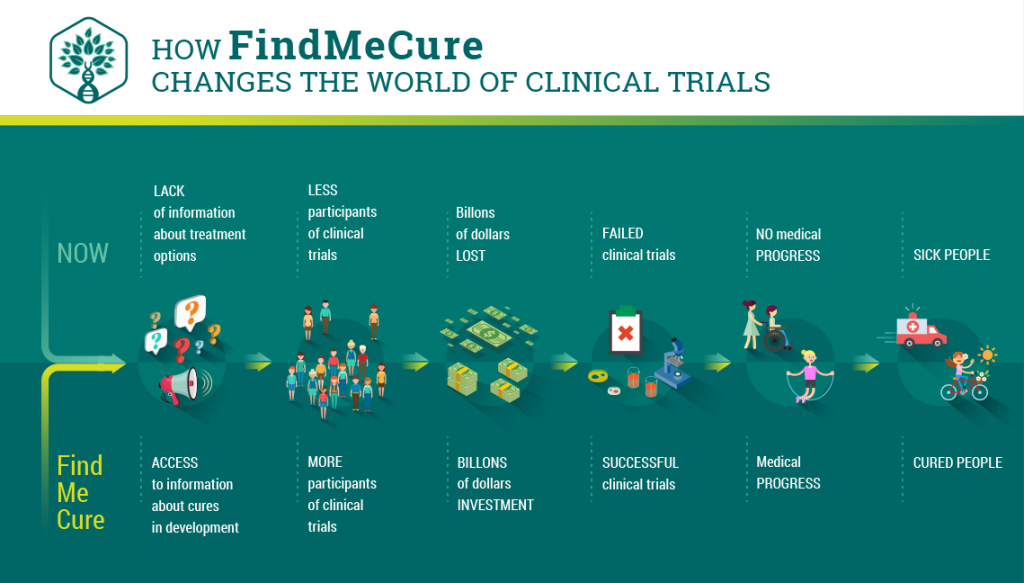
Nowadays, FindMeCure team is here advocating for affordable and accessible health care for all. Currently, there are over 50 000 available clinical trials researching new drugs, innovative treatments, and medical devices. Each of those is a potential new medicine. However, none could be in use for you unless you know how to find them, choose the right one and apply for participation. FindMeCure is here to help you. We provide a free access to more than 90% of the clinical trials worldwide. With us, you can search, find, apply and participate in the right clinical trials for you within few clicks.


Thanks for the interesting glance at history. You are doing great work.