The 5 Most Popular Myths About New Drugs — Debunked (Infographic)

Find Your TrialOur co-founder Maya is an outstanding speaker, but also a great writer. In this article, she is sharing some insights on clinical trials as well as her own experience on using new treatments. Maya has worked within the clinical research industry for almost 10 years now. She has delivered training and software solutions for organizations like Takeda, Roche, Ministries of Health, the United Nations etc.
Today her inspiration and efforts are powered by and focused on FindMeCure – the Google of clinical trials.
In every industry, there are series of myths that lay in our minds and prevent us from taking all the advantages that a certain area can give us. With this article, we are sharing 5 myths from the drug development industry that can broaden your perspective and help you make more informed decisions in the future.
If you like this article, we would love to get your recommendation and shares so that others can expand their knowledge too.
1. Drug development focuses only on chemical formulas for medicines — FALSE
When we speak about the drug making industry we imagine only the actual drugs or otherwise said the pills we are usually buying from a pharmacy. The healthcare sector needs different approaches to treat people and so the drug development actually includes a lot of research not only on chemical formulas but also on medical devices and treatment procedures.
When I first started reading about clinical trials and using them as an alternative way to find a cure for my sister, who had bulimia, I was shocked to find out that among the many drugs tested for eating disorders, there was one clinical trial, organized by an Israeli pharma company, which used Yoga for treatment.
There are many other examples of how medical progress can provide us with a lot more options than just pills and I am glad that with the help of technology there are now things like nanobots fighting cancer, 3-D printing of organs, exoskeletons etc.
2. All drugs on the market are 100% proven to be efficient — FALSE
Мost of the drugs on the market have been used for centuries and are well known by their advantages and disadvantages. This is not the case with drugs that are just coming to the market, or scientifically said drugs in a Post-Marketing stage. You can learn more about the Stages of Drug Development using this infographic:
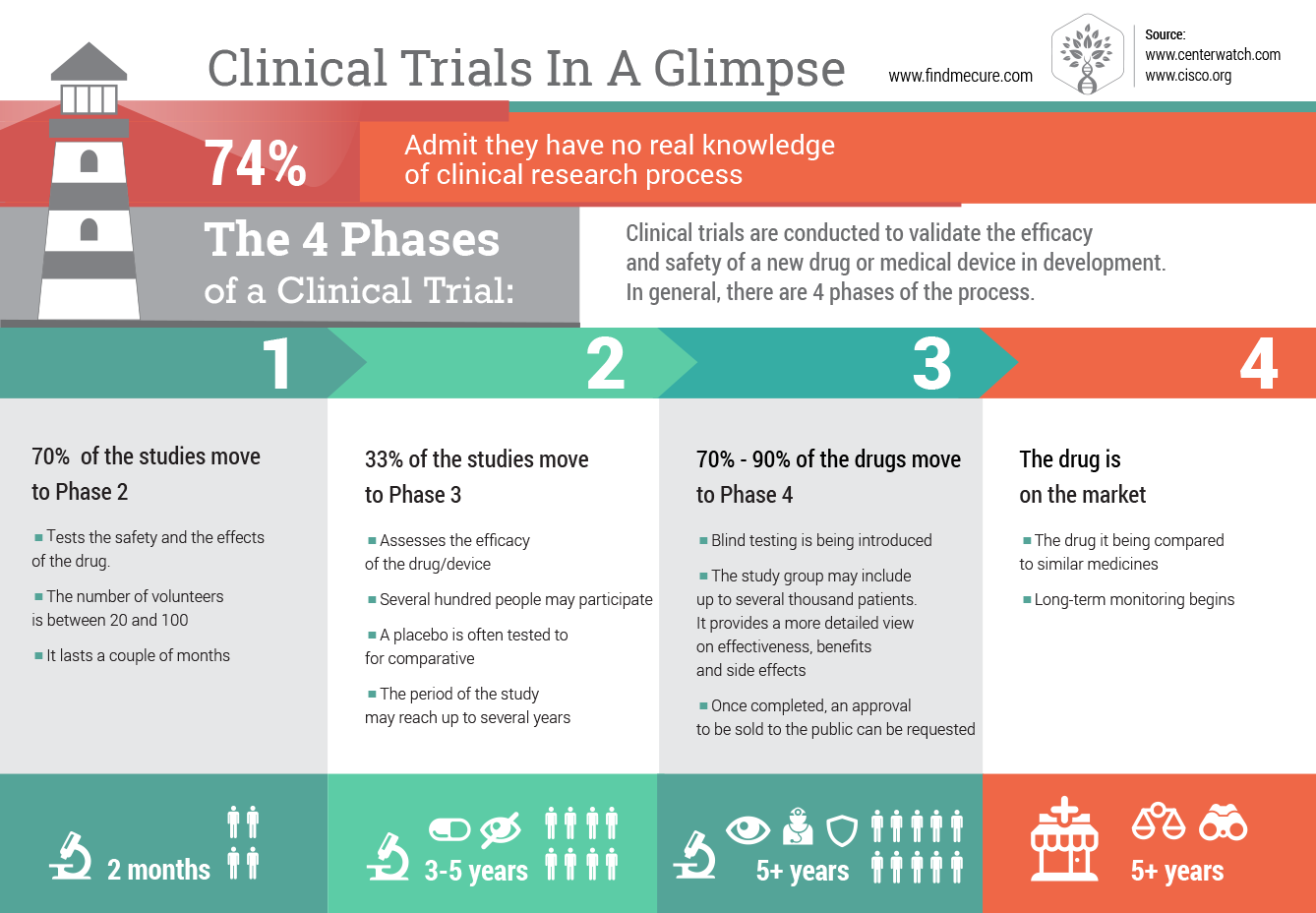
Drugs in the 4th stage are usually thoroughly tested by thousands of people worldwide and the way they act on the human body has been recorded and documented by several years of clinical research. But it is also well known that we humans are all different and what can work to one is not 100% sure to be working on another.
I had an interesting case with such a drug. It was in the years when I was still not aware of what actually clinical trials are and how they work. So, one day I visited my doctor who prescribed me contraceptives to prevent unwanted pregnancy.
He said that these drugs are quite new, very well accepted and they have shown minimum side effects. I just had to wait a month or so to get used to them.
Which I did…3 months later I was on the edge of emotional crisis, had no period and I didn’t know where all this is coming from because this was not the first time I was taking similar pills. But this time it was so bad, even my mother asked me to stop taking them.
And I did, I went to another doctor, shared my case and then I found out that this drug is absolutely new to the market in Post-Marketing stage and though many women really liked it, I was one of the cases that had some serious side effects and had to stop it.
The good thing here is that there are publically available registries (usually in every country, but also attached to regional institutions or even private companies) where people can report side effects they observe. This way the more people share their experiences, the better the drugs can be used and prescribed.
3. Drugs with different names have all different compounds — FALSE
Another myth from the pharma world: Many of the drugs have the same or really little difference in their molecular formulas. The difference comes from the way they are marketed, their name, price and producing company.
There are even cases when one and the same drug formula has been marketed by the same company with different names to make it more marketable and increase prices. Such an example are Eli Lillys’ Prozac and Sarafem.
Prozac was an extremely successful drug researched and marketed by the company. Once Eli Lilly had to share the patent with other organizations and to fight the completion mainly by price, Sarafem was released — the same formula drug but advertised as the pills that help women fight their premenstrual dysphoric disorder and other symptoms.
That way the pink pill was able to keep the interest and most importantly keep the price of the product.
4. A drug by the same name has same compounds in every country — FALSE
One of the ways clinical trials make sure that a drug really works is by testing the chemical formula on different groups of people also divided by regions. In Phase 3 when it has proven a lot of positive results the drug also needs to be researched in different countries.
A reason for this is that each region or country has a specific way of life, food diet and activities that can increase or decrease the effect of the chemical combination.
This is also why in many cases one and the same drug can have different compounds in different countries. An example for that is the widespread Aspirin, registered trademark of Bayer. Of course the difference is really small, but still there is.
5. Drug making is a secret process no one knows anything about — FALSE
The good news is that the drug development is not really a secret. Since World War II and the terrifying tests on humans, the clinical research has become an official process following serious series of international and local regulations similar to other industries like the airline industry. (For more details you can read about the development of the first rules in clinical research here: http://www.ich.org ).
Nowadays every single person involved in a clinical trial has to be trained and certified in ICH -GCP (Good Clinical Practice) as these are the official guidelines of how a clinical trial should be designed, performed, recorded and documented to ensure the safety of all patients.
According to one of the regulations there all drugs in development must meet the requirements of the governmental institutions like FDA in the USA or MHRA in the UK or BfarM in Germany etc. and have to be approved before entering the market.
During the research, all their documentation has to be submitted and part of it officially published in public clinical trial registries like www.clinicaltrials.gov or www.ema.europe.eu etc.
So, everyone can be aware of what’s coming and what to expect or which compounds are still being tested.
The trouble is that not everyone knows how to read and understand this data.
So here FindMeCure comes. We help you search and find the most suitable clinical trials for you based on your condition and treatment preferences. It’s quick, it’s easy, it’s free. Try it yourself by using the search field below or start a fresh search here.
*article originally published on Medium


[…] Read “The 5 Most Popular Myths About New Drugs — Debunked (Infographic)” […]